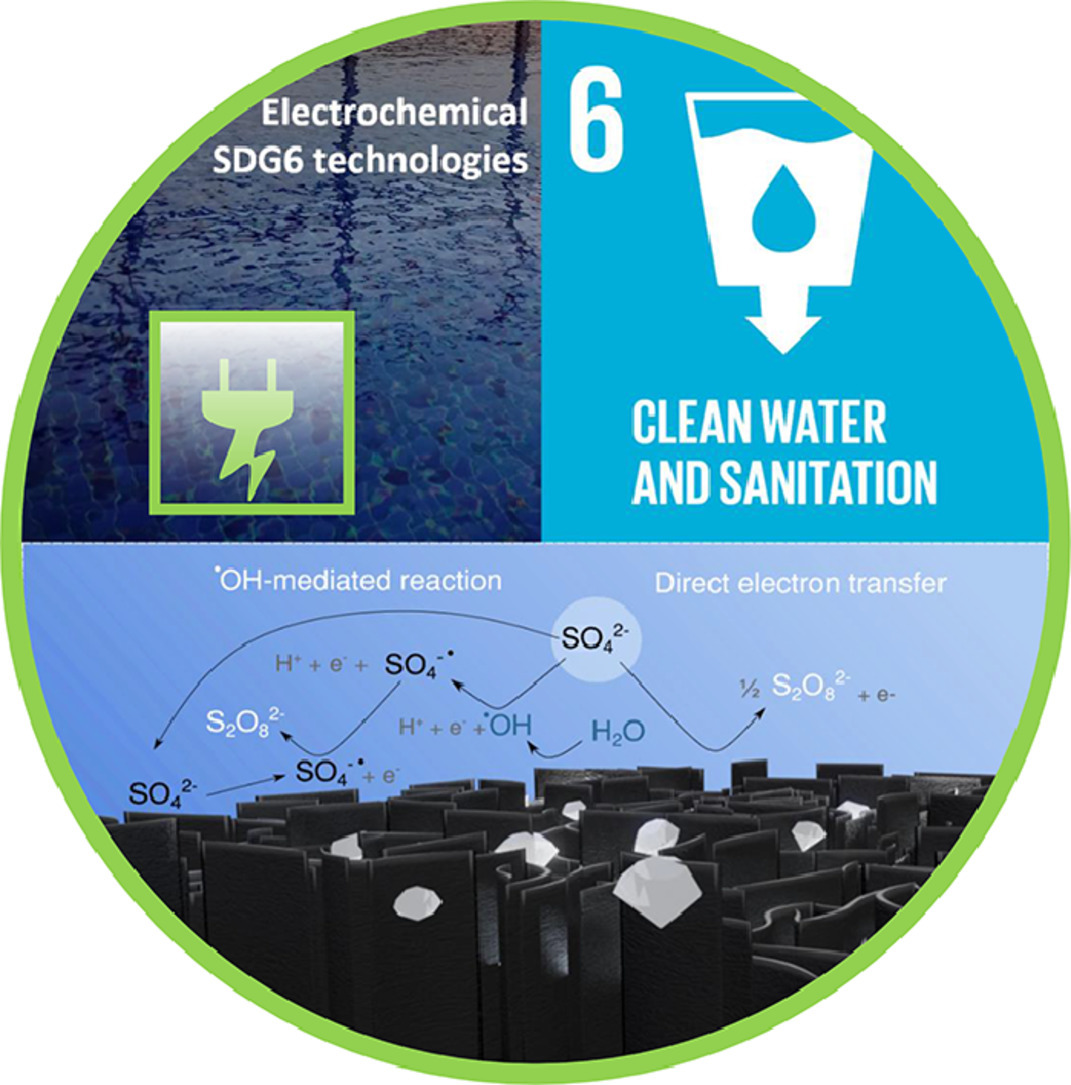
Diamondized carbon nanoarchitectures as electrocatalytic material for sulfate-based oxidizing species electrogeneration
The introduction of nanotechnology seems to be an imperative factor to intensify the synergic effects of electrocatalytic materials to produce strong oxidant species or to increase the active sites on their surfaces as well as to enhance the conversion yield in a fuel cell, high-added value products, electrolytic treatment for environmental protection or the detection limit in electroanalysis. Recently, a new type of 3D-diamond electrodes was developed with boron-doped carbon nanowalls (B:CNW), which was manufactured using the microwave plasma-assisted chemical vapor deposition (CVD) process, improving the charge transfer and enhancing the electrochemical performance. The applicability of a BDD/boron-doped carbon nanowalls (BDD/B:CNW) anodes to degrade organic pollutants has been already investigated; however, no attempts at the electrosynthesis of oxidizing species using these diamond-carbon nanostructures have been reported yet. Therefore, the electrosynthesis of sulfate-based oxidizing species was studied here to answer relevant questions from both fundamental and practical point-of-view. The results demonstrated that persulfate was efficiently produced at the BBD electrode, while that the ion-radical sulfate could be the most important oxidant at BDD/B:CNW anode when compared to other electrocatalytic materials, including BDD surfaces. Persulfate concentrations ranged from 3 to 6 µM, depending on the applied current density (2.5, 5.0, and 15 mA cm−2), at diamond electrodes. A dye-model pollutant - methyl orange (MO) was degraded below the limit of detection within 45 min using BDD/B:CNW when in-situ sulfate-based oxidizing species were electrogenerated. These kinds of 3D-diamond-carbon nanostructures are thus promising as novel electrocatalyst for various catalytic applications in the environmental and energy fields.